What is Thermodynamic System?
A thermodynamic system is defined as a fixed mass in space under thermodynamic consideration to analyse a problem.
The system is identified by a boundary drawn around the system which may be real or imaginary. Across the boundary, the energy transfer in the form of heat and work takes place.
Shape, volume, the position of the boundary may change during energy exchange with the surroundings.
Everything external to the system is called surroundings or environment.
 |
| Thermodynamic System |
A system and its surroundings together are called the universe.
Classification of Thermodynamic Systems
Many times it is asked, to classify and explain the classification of the thermodynamic system.
Based on the mass and energy transfer between the system and the surrounding, the thermodynamic system can be classified as
- Open system.
- Closed system.
- Isolated system.
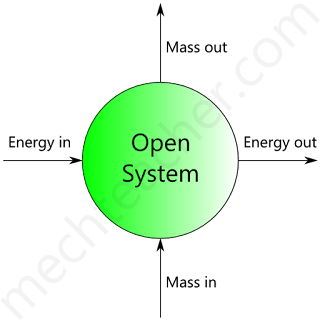
Open system
A system with mass transfer along with energy transfer across its boundaries is called an open system.
Fig below shows an open system that consists of a turbine.
 |
| Open System |
It should be noted that the matter across the boundary of the system as the high-pressure gases enter into the turbine, low-pressure gases enter into the turbine and low-pressure gases leave the turbine.
Also, it is not necessary that the quantity of matter within the boundaries of an open system to remain fixed.
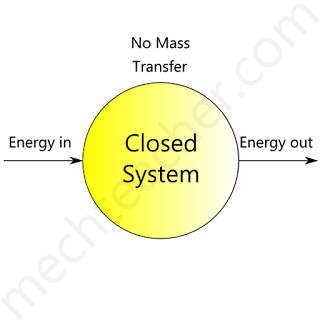
Closed system
A system without mass transfer across its boundaries is called a closed system.
Such systems have only the energy transfer in the form of heat and work with their surroundings across the system boundary.
The figure below shows a closed system.
 |
| Closed System |
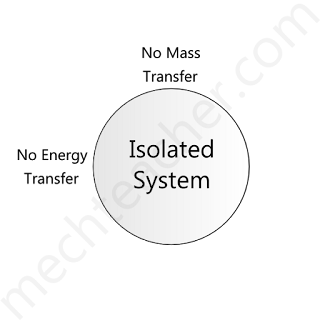
Isolated system
If there is no mass and energy transfer between the system and surroundings, the system is said to be an isolated system.
Hence, according to the definition, the universe is an isolated system.
A handy example of an isolated system is a thermos flask.
The figure below shows an isolated system.
 |
| Isolated System |








Amazing things here. I am very satisfied to look your post.
Thank you a lot and I am looking forward to touch you.
Will you kindly drop me a e-mail?
Undeniably believe that which you said. Your favorite justification appeared to be on the web the easiest factor to have in mind of.
I say to you, I definitely get annoyed even as other people consider concerns that they plainly
do not know about. You controlled to hit the nail upon the highest and outlined out the whole thing with no need side effect , other people could
take a signal. Will likely be again to get more.
Thank you
Thanks very nice blog!
I’ve learn several good stuff here. Certainly value bookmarking for revisiting. I wonder how so much effort you put to make any such excellent informative web site.
Generally I don’t read post on blogs, however I wish to say that this write-up very
compelled me to take a look at and do so! Your writing style
has been amazed me. Thanks, very great article.
Thank you for another fantastic article. Where else may just anybody get
that kind of information in such an ideal approach of writing?
I have a presentation subsequent week, and I’m on the search for
such info.
I really like it when people come together and share ideas.
Great blog, stick with it!
Magnificent items from you, man. I have bear in mind your stuff prior to and you are just extremely excellent.
I really like what you’ve bought here, really like what you’re
saying and the way by which you say it. You are making it
enjoyable and you continue to care for to stay it sensible.
I cant wait to read far more from you. This
is actually a wonderful web site.
Awesome things here. I’m very satisfied to peer your article.
Thanks a lot and I am looking ahead to touch you.
Will you kindly drop me a e-mail?